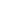EGF信号:追踪癌症的路径
发表时间:2022-12-05EGF信号:追踪癌症的路径
表皮生长因子(EGF)家族是一组结构相关的蛋白质,通过靶细胞上的酪氨酸激酶受体调节细胞增殖、迁移和分化。EGF受体有一个细胞质酪氨酸激酶结构域,一个跨膜结构域和一个与EGF结合的细胞外结构域。配体与EGF受体结合导致其二聚、自磷酸化和激活。一旦被激活,EGF受体通过几个蛋白质的磷酸化传递细胞内信号。
Ras被EGF受体激活是EGF信号转导的重要组成部分。鸟嘌呤核苷酸交换因子SOS激活Ras, Ras进而触发丝裂原激活蛋白(MAP)激酶通路。MAP激酶磷酸化转录因子,如激活蛋白1(AP-1;Fos-Jun二聚体)和Elk-1,导致细胞生长和发育。EGFR对Janus激酶(JAK)的磷酸化导致转录蛋白信号换能器和激活器(STATs)的激活,最终导致细胞的生长和分化。EGF信号的另一个关键方面涉及磷脂酶c - γ1 (PLCγ1),它将PIP2裂解为IP3和DAG。IP3的产生导致内质网钙的释放,而DAG促进蛋白激酶C (PKC)的激活。PKC反过来磷酸化并激活转录因子Elk-1,导致细胞增殖。已知EGFR的突变影响其表达或活性,这使EGFR成为重要的药物靶点。
该通路强调了EGF信号转导的重要组成部分。
几个重要的表皮生长因子信号通路
|
基因符号 |
名称 |
细胞功能 |
关联疾病 |
亚细胞定位 |
上游调节 |
结合伙伴 |
下游作用 |
抗体 |
小分子 |
|
STAT3 |
信号换能器和转录激活器3(急性期反应因子) |
扩散 细胞凋亡 表达 转换 分化 |
肿瘤发生 肥胖 小肠结肠炎 克罗恩氏病 食欲过盛 |
核 细胞质 焦粘连 核焦点 等离子体膜 |
IL6 IL10 IL2 IL21 α-干扰素 |
FOS EGFR PRKCD DIRAS3 IL2RB |
TERT IL10 HIF1A CDKN1A SOCS3 |
抑制STAT3的抗体,来源于山羊 |
|
|
EGFR |
表皮生长因子受体 |
扩散 细胞凋亡 迁移 转换生存 |
癌症 肿瘤发生 瘤形成 牛皮癣 子宫内膜异位 |
细胞表面 等离子体膜 核 细胞质 小窝 |
EGF TNF CBL 溶血磷脂酸HBEGF |
EGF AXL GRB2 CBL SRC |
Mapk MAPK1 Akt Erk1/2 MAPK3 |
单克隆抗表皮生长因子抗体,来源于鼠 |
抑制剂: PZ0129 CP-380736 |
|
c-Raf |
V-raf-1小鼠白血病病毒癌基因同源物1 |
转换 扩散 细胞凋亡 细胞周期进程 细胞死亡 |
转换扩散 细胞凋亡 细胞周期进程 细胞死亡 |
细胞质 核 细胞核周围的地区 等离子体膜 丝状网络 |
EGF TP53 JAK1 OSM HRAS |
HRAS YWHAZ MAP2K1 YWHAB RB1 |
MAPK1 RB1 HMGA2 MAP2K1 Mapk |
单克隆anti-Raf-1/c-Raf抗体,来源于鼠 |
抑制剂: G6416, GW5074
|
|
c-Jun |
jun原癌基因 |
细胞凋亡 扩散 转换 细胞死亡中的表达 |
肿瘤发生 癌症 瘤形成 阿尔茨海默病 去分化 |
核 细胞质 细胞核周围的地区 高尔基体 顶端的过程 |
TNF IL1B β-雌二醇 TGFB1 脂多糖 |
FOS PTGS2 MAPK8 TAF1 ATF2 |
HIF1A SPP1 IL6 IL8 ESR2 |
Anti-JUN(Ab-91)抗体,来源于兔 |
|
|
PKCα |
蛋白激酶C,α |
细胞凋亡 扩散 激活 迁移 磷酸化 |
神经退行性疾病 糖尿病 类风湿性关节炎 恶性肿瘤 心肌病 |
细胞质核 等离子体膜 主段 细胞骨架 |
磷脂酰丝氨酸 EGF β-雌二醇 15(S)-HETE D-葡萄糖 |
ITGB1 AKAP12 EGFR CAV1 SELL |
Erk1/2 APP PDE3A MAPK1 IGF2 |
单克隆Anti-PRKCA抗体,来源于鼠 |
抑制剂: K1639, K252a |
|
STAT1 |
信号转换器和转录激活器1,91kDa |
细胞凋亡 表达 扩散 响应 分化 |
感染 肿瘤发生 肺炎 癌症 纤维化 |
核 细胞质 线粒体 神经肌肉 连接 |
IFNG 干扰素 IFNA2 IL6 IFNB1 |
EIF2AK2 IFNGR1 FOS STAT2 PIN1 |
IRF1 CDKN1A IRF7 CASP1 CD40 |
Anti-STAT1(Ab-701)抗体,来源于兔 |
|
|
EGF |
表皮生长因子 |
扩散 迁移 细胞凋亡 增长 激活 |
阿尔茨海默病 糖尿病 多囊肾疾病 精神分裂症 癌症 |
顶端膜 基底膜 细胞表面 高尔基体 clathrin-coated 囊泡 |
ERBB2 ERBB3 ADAM10 CHUK PI4KA |
EGFR ERBB3 ERBB2 PIK3R2 TAT |
EGFR MAPK1 MAPK3 FLT1 Erk1/2 |
抑制剂: S2671, 苏拉明钠盐 |
|
|
GRB2 |
生长因子受体结合蛋白2 |
增长 扩散 分化 信号 转换 |
克罗恩氏病 平滑肌瘤病心脏纤维化 肥大 子宫癌 |
中心体 胞质 细胞核周围的地区 等离子体膜 轴突 |
F2 EGF Bcr IGF1 SHC1 |
SHC1 CBL SOS1 EGFR GAB1 |
MAPK3 EGFR ERBB2 RAF1 CBL |
Anti-GRB2抗体,来源于山羊 |
|
|
MEK1 |
丝裂原激活蛋白激酶激酶1 |
细胞凋亡 扩散 转换 分化 迁移 |
肿瘤发生 瘤形成 肥大 心脸皮肤综合征 并发症状 痛觉过敏 |
细胞质 中体 核 中心体 有丝分裂纺锤体 |
EGF LEF RAF1v RAC1 TNF |
MAPK1 RAF1 MAPK3 PEBP4 KSR1 |
MLANA MAPK1 DCT SILV TYRP1
|
Anti-MEK1抗体,来源于兔 |
抑制剂: P215, PD98,059 |
|
hRas |
v-Ha-ras哈维鼠肉瘤病毒癌基因同源物 |
转换 扩散 增长 细胞凋亡 衰老 |
肿瘤发生 癌症 瘤形成 乳头瘤病 神经退化 |
核 等离子体膜 高尔基体 细胞质 细胞变形足 |
Cd3 CD28 AXIN1 FTase IL6 |
RAF1 RALGDS RIN1 Blnk Ra |
活性氧 CDKN1A MAPK1 Erk1/2 Mapk |
Anti-RASH,N封端抗体,来源于兔 |
拮抗剂: E7781, Erastin |
|
c-Fos |
FBJ小鼠骨肉瘤病毒癌基因同源物 |
转换 细胞凋亡 扩散 表达 增长 |
癌症 类风湿性关节炎 子宫内膜异位瘤形成 癫痫发作 |
核 细胞质 细胞核周围的地区 高尔基体 细胞外围 |
β-雌二醇 TNF IL1B EGF ESR2 |
JUN STAT3 PTGS2 SRF IL8 |
IL6 CSF2 ESR2 IL8 CFLAR |
Anti-FOS抗体,来源于兔 |

参考文献
1. Corbalan-Garcia S, Margarit SM, Galron D, Yang S, Bar-Sagi D. 1998. Regulation of Sos Activity by Intramolecular Interactions. Mol. Cell. Biol.. 18(2):880-886. https://doi.org/10.1128/mcb.18.2.880
2. Russell M, Lange-Carter CA, Johnson GL. 1995. Direct Interaction between Ras and the Kinase Domain of Mitogen-activated Protein Kinase Kinase Kinase (MEKK1). Journal of Biological Chemistry. 270(20):11757-11760. https://doi.org/10.1074/jbc.270.20.11757
3. Ackerman P, Glover CV, Osheroff N. 1990. Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit.. Proceedings of the National Academy of Sciences. 87(2):821-825. https://doi.org/10.1073/pnas.87.2.821
4. Andl CD, Mizushima T, Oyama K, Bowser M, Nakagawa H, Rustgi AK. 2004. EGFR-induced cell migration is mediated predominantly by the JAK-STAT pathway in primary esophageal keratinocytes. American Journal of Physiology-Gastrointestinal and Liver Physiology. 287(6):G1227-G1237. https://doi.org/10.1152/ajpgi.00253.2004
5. Baker SJ, Kerppola TK, Luk D, Vandenberg MT, Marshak DR, Curran T, Abate C. 1992. Jun is phosphorylated by several protein kinases at the same sites that are modified in serum-stimulated fibroblasts.. Mol. Cell. Biol.. 12(10):4694-4705. https://doi.org/10.1128/mcb.12.10.4694
6. Takekawa M, Tatebayashi K, Saito H. 2005. Conserved Docking Site Is Essential for Activation of Mammalian MAP Kinase Kinases by Specific MAP Kinase Kinase Kinases. Molecular Cell. 18(3):295-306. https://doi.org/10.1016/j.molcel.2005.04.001
7. Chong M, Barritt G, Crouch M. 2004. Insulin potentiates EGFR activation and signaling in fibroblasts. Biochemical and Biophysical Research Communications. 322(2):535-541. https://doi.org/10.1016/j.bbrc.2004.07.150
8. Krug AW, Schuster C, Gassner B, Freudinger R, Mildenberger S, Troppmair J, Gekle M. 2002. Human Epidermal Growth Factor Receptor-1 Expression Renders Chinese Hamster Ovary Cells Sensitive to Alternative Aldosterone Signaling. Journal of Biological Chemistry. 277(48):45892-45897. https://doi.org/10.1074/jbc.m208851200
9. Lim CP, Cao X. 1999. Serine Phosphorylation and Negative Regulation of Stat3 by JNK. Journal of Biological Chemistry. 274(43):31055-31061. https://doi.org/10.1074/jbc.274.43.31055
10. Diakonova M, Payrastre B, van Velzen AG, Hage W, van Bergen en Henegouwen PM, Boonstra J, Cremers F, Humbel B. 1995. Epidermal growth factor induces rapid and transient association of phospholipase C-gamma 1 with EGF-receptor and filamentous actin at membrane ruffes of A431 cells.. J Cell Sci..(108):2499–2509.
11. Eldar H, Zisman Y, Ullrich A, Livneh E. 1990. Overexpression of protein kinase C alpha-subtype in Swiss/3T3 fibroblasts causes loss of both high and low affinity receptor numbers for epidermal growth factor.. Journal of Biological Chemistry. 265(22):13290-13296. https://doi.org/10.1016/s0021-9258(19)38297-3
12. Weston CR, Wong A, Hall JP, Goad MEP, Flavell RA, Davis RJ. 2004. The c-Jun NH2-terminal kinase is essential for epidermal growth factor expression during epidermal morphogenesis. Proceedings of the National Academy of Sciences. 101(39):14114-14119. https://doi.org/10.1073/pnas.0406061101
13. Carpenter G, Cohen S. 1990. Epidermal growth factor.. Journal of Biological Chemistry. 265(14):7709-7712. https://doi.org/10.1016/s0021-9258(19)38983-5
14. Hu, Bowtell D. 1996. Sos1 rapidly associates with Grb2 and is hypophosphorylated when complexed with the EGF receptor after EGF stimulation. Oncogene.. 12(9):1865–72.
15. Ueno H, Sasaki K, Miyagawa K, Honda H, Mitani K, Yazaki Y, Hirai H. 1997. Antisense Repression of Proto-oncogene c-Cbl Enhances Activation of the JAK-STAT Pathway but Not the Ras Pathway in Epidermal Growth Factor Receptor Signaling. Journal of Biological Chemistry. 272(13):8739-8743. https://doi.org/10.1074/jbc.272.13.8739
16. Cummins AB, Palmer C, Mossman BT, Taatjes DJ. 2003. Persistent Localization of Activated Extracellular Signal-Regulated Kinases (ERK1/2) Is Epithelial Cell-Specific in an Inhalation Model of Asbestosis. The American Journal of Pathology. 162(3):713-720. https://doi.org/10.1016/s0002-9440(10)63867-9
17. Yoshikawa S, Tanimura T, Miyawaki A, Nakamura M, Yuzaki M, Furuichi T, Mikoshiba K. 1992. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster.. Journal of Biological Chemistry. 267(23):16613-16619. https://doi.org/10.1016/s0021-9258(18)42047-9
18. Mahimainathan L, Ghosh-Choudhury N, Venkatesan BA, Danda RS, Choudhury GG. 2005. EGF stimulates mesangial cell mitogenesis via PI3-kinase-mediated MAPK-dependent and AKT kinase-independent manner: involvement of c-fos and p27Kip1. American Journal of Physiology-Renal Physiology. 289(1):F72-F82. https://doi.org/10.1152/ajprenal.00277.2004
19. Xia Y, Makris C, Su B, Li E, Yang J, Nemerow GR, Karin M. 2000. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proceedings of the National Academy of Sciences. 97(10):5243-5248. https://doi.org/10.1073/pnas.97.10.5243
20. Chen D, Davis JS. 2003. Epidermal growth factor induces c-fos and c-jun mRNA via Raf-1/MEK1/ERK-dependent and -independent pathways in bovine luteal cells. Molecular and Cellular Endocrinology. 200(1-2):141-154. https://doi.org/10.1016/s0303-7207(02)00379-9